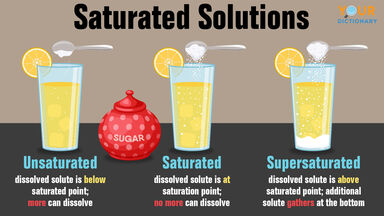
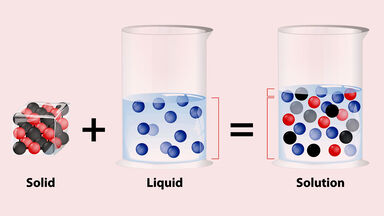
Solute Definition
sŏlyo͝ot, sōlo͝ot
solutes
noun
The substance dissolved in a solution.
Webster's New World
adjective
Being in solution; dissolved.
American Heritage
Loose; free; liberal.
A solute interpretation.
Wiktionary
Relaxed; hence; merry; cheerful.
Wiktionary
A solute salt.
Wiktionary
(botany) Not adhering; loose; opposed to adnate.
A solute stipule.
Wiktionary
Other Word Forms of Solute
Noun
Singular:
solutePlural:
solutesFind Similar Words
Find similar words to solute using the buttons below.