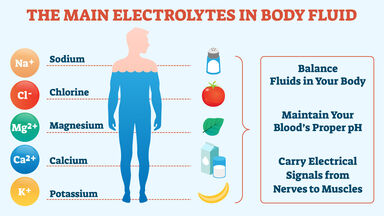
Sodium Definition
sōdē-əm
noun
A soft, silver-white, metallic chemical element, one of the alkali metals, having a waxlike consistency: it is found in nature only in combined form and is extremely active chemically: symbol, Na; at. no. 11
Webster's New World
Webster's New World
A soft, waxy, silvery reactive metal that is never found unbound in nature, and a chemical element (symbol Na) with an atomic number of 11 and atomic weight of 22.98977.
Wiktionary
Synonyms:
- na
- atomic number 11
Other Word Forms of Sodium
Noun
Singular:
sodiumPlural:
sodiumsOrigin of Sodium
-
Coined by Humphry Davy in 1807, from soda.
From Wiktionary
sod(a) –ium
From American Heritage Dictionary of the English Language, 5th Edition
Find Similar Words
Find similar words to sodium using the buttons below.