Ion Exchange Definition
noun
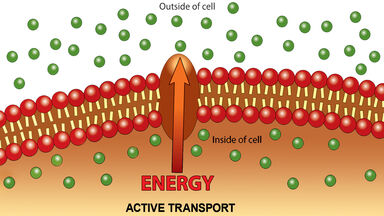
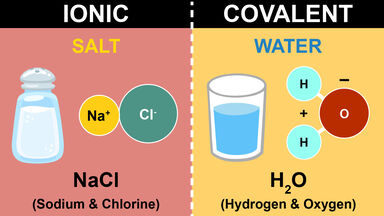
A chemical process whereby ions are reversibly transferred between an insoluble solid, such as a resin, and a fluid mixture, usually an aqueous solution: widely used in water softening, in recovering metals from waste solutions, etc.
Webster's New World
Other Word Forms of Ion Exchange
Noun
Singular:
ion exchangePlural:
ion exchangesFind Similar Words
Find similar words to ion exchange using the buttons below.