Hydrogen Peroxide Definition
noun
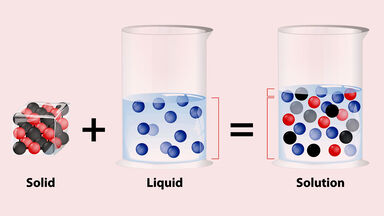
A colorless, syrupy liquid, H2O2, often used in dilute, unstable solutions as a bleaching or disinfecting agent, and in more concentrated form as a rocket fuel, in the production of foam rubber, etc.
Webster's New World
Synonyms:
Hydrogen Peroxide Is Also Mentioned In
Find Similar Words
Find similar words to hydrogen peroxide using the buttons below.