Formic Definition
fôrmĭk
adjective
Of ants.
Webster's New World
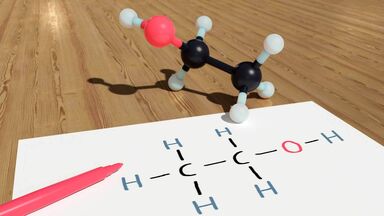
Designating or of a colorless acid, HCOOH, that is extremely irritating to the skin: it is found in living organisms, as ants, spiders, and nettles, and is prepared commercially for use in dyeing textiles, treating leather, preserving food, etc.
Webster's New World
Of, derived from, or containing formic acid.
American Heritage Medicine
Find Similar Words
Find similar words to formic using the buttons below.