Electrolyte Definition
ĭ-lĕktrə-līt
electrolytes
noun
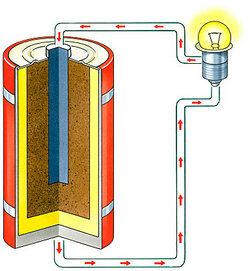
Any chemical compound that ionizes when molten or in solution, allowing it to conduct electricity.
Webster's New World
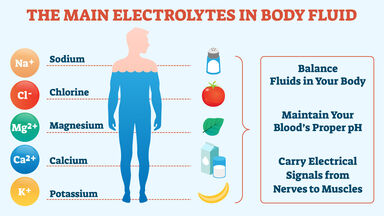
Any ionized compound, as of sodium, potassium, or calcium, essential in maintaining certain metabolic processes.
Webster's New World
Other Word Forms of Electrolyte
Noun
Singular:
electrolytePlural:
electrolytesFind Similar Words
Find similar words to electrolyte using the buttons below.