Specific Heat Definition
noun
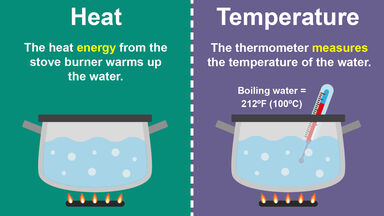
The ratio of the amount of heat required to raise the temperature of a given mass of a substance one degree to the amount of heat required to raise the temperature of an equal mass of water one degree.
Webster's New World
The number of calories needed to raise the temperature of one gram of a given substance 1°C.
Webster's New World
Other Word Forms of Specific Heat
Noun
Singular:
specific heatPlural:
specific heatsRelated Articles
Specific Heat Is Also Mentioned In
Find Similar Words
Find similar words to specific heat using the buttons below.