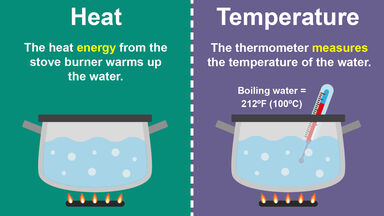
In SI units the specific heat capacity is the amount of heat required to raise 1 kg mass through 1 degree Kelvin.
Neumann, who, in 1831, deduced from observations on many carbonates (calcium, magnesium, ferrous, zinc, barium and lead) that stoichiometric quantities (equimolecular weights) of compounds possess the same heat capacity.
This Variation May Have Been Due To The State Of The Lagging, Which Moorby Distrusted In Spite Of The Great Reduction Of The Heat Loss, Or It May Have Been Partly Due To The Difficulty Of Regulating The Speed Of The Engine And The Watersupply To The Brake In Such A Manner As To Maintain A Constant Temperature In The Outflow, And Avoid Variations In The Heat Capacity Of The Brake.
The white storage elements consist of a highly heat resistant material based on special fused alumina which has very high heat capacity and conductivity.
The enthalpy values were obtained by integrating the specific heat capacity polynomial for each compound.