Ionic Bond Definition
ī-ŏnĭk
noun
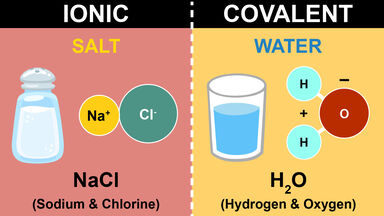
The chemical bond between two oppositely charged ions formed when one atom transfers electrons to another atom, as in the formation of sodium chloride; electrovalent bond.
Webster's New World
Synonyms:
- electrostatic bond
- electrovalent bond
Other Word Forms of Ionic Bond
Noun
Singular:
ionic bondPlural:
ionic-bondsIonic Bond Is Also Mentioned In
Find Similar Words
Find similar words to ionic bond using the buttons below.