Coordinate Bond Definition
noun
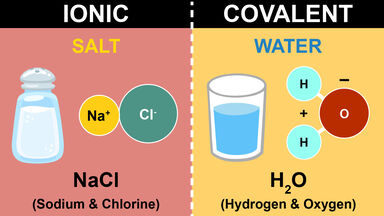
The type of covalent bond in which the shared pair of electrons is supplied by only one of the atoms; dative bond.
Webster's New World
Synonyms:
Other Word Forms of Coordinate Bond
Noun
Singular:
coordinate bondPlural:
coordinate-bondsFind Similar Words
Find similar words to coordinate bond using the buttons below.