Proton Definition
prōtŏn
protons
noun
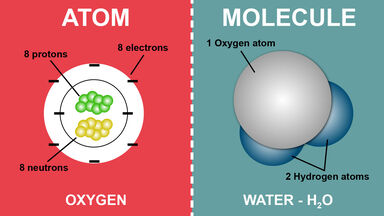
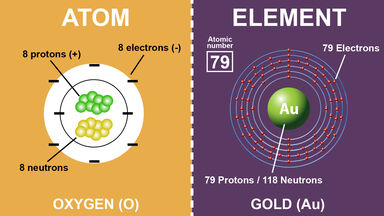
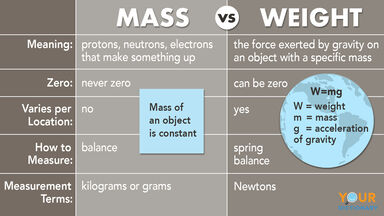
A nucleon carrying a positive charge equal to the negative charge of an electron and having a mass of c. 1.673 × 10-27 kg (c. 938.2796 MeV/c2, c. 1,836 times the mass of an electron): the number of protons in an atom determines the atomic number of a chemical element.
Webster's New World
Other Word Forms of Proton
Noun
Singular:
protonPlural:
protonsOrigin of Proton
-
From Greek prōton neuter of prōtos first per1 in Indo-European roots
From American Heritage Dictionary of the English Language, 5th Edition
From Ancient Greek πρῶτον (prōton), neuter of πρῶτος (prōtos, “first")
From Wiktionary
Related Articles
Find Similar Words
Find similar words to proton using the buttons below.